
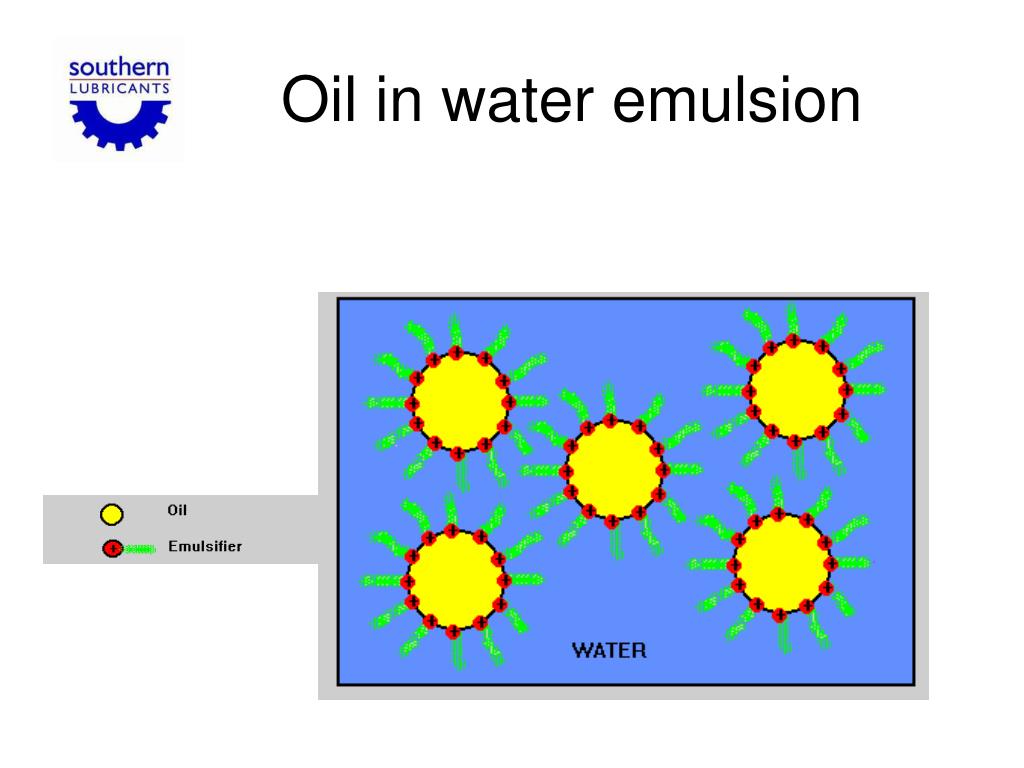
In this work, we investigate droplet coalescence in flowing oil-in-water emulsions subjected to higher than room temperatures namely between 20 to 70 \(^\). In their preparation, combinations of fatty. The best example of w/o emulsion is Margarine (a spread used for flavouring, baking, and. In Water in the oil (w/o) type, water will be the dispersed phase and oil will be the dispersion medium. The crucial impact of certain conditions such as increased pressure or elevated temperature frequently used in industrial processes is completely overlooked in such micro-scale studies. Synopsis Cosmetic oil-in-water emulsions such as lotions and creams are complex multiple-phase systems. The best example of o/w emulsion is milk in which the fat globules (act as the dispersed phase) are suspended in water (act as the dispersion medium). Microfluidic studies can provide micro-scale insight of the emulsion behavior but have primarily focussed on droplet breakup rather than on droplet coalescence. The emulsifier molecule creates a ‘bridge’ that attracts water and non-water molecules and enables them to be held close together and not separate.ĭepending on the emulsifier and the quantities used for each water and oil component, emulsions will contain tiny droplets of one of the liquids spread through the other liquid. For example, proteins dissolve better in water than in oil, and so tend to form oil-in-water emulsions (that is, they promote the dispersion of oil droplets throughout a continuous phase of water).Emulsion stability in a flow field is an extremely important issue relevant for many daily-life applications such as separation processes, food manufacturing, oil recovery etc. Emulsifiers are molecules that contains two different binding sites: hydrophilic (prefer ‘water’ and binds to water molecules) and hydrophobic(avoids ‘water’ and binds to oil molecules). In this role, the micelle is known as a plasma lipoprotein. The phospholipid emulsifies the fat molecules held inside the micelle. A spherical micelle molecule formed by a ring of phospholipids (green) that allows cholesterol/triglyceride fats (inside yellow zone) to be carried in the blood stream (which is mainly plasma water). Classification, variation, food webs and pyramids.Combustion reactions and impact on climate.Atoms elements compounds and mixtures (interactive).B1.6 Waste materials from plants and animals.A stable emulsion requires an emulsifying agent to be present. An emulsion is a colloidal dispersion of a liquid in either a liquid or a solid. RSC Learn Chemistry Classic Chemistry Experiments Butter and mayonnaise are examples of a class of colloids called emulsions.The most common emulsions are oilwater systems, which have found widespread use across a number of industries, for example, in the cosmetic and food industries, and are also of advanced scientific interest.

RSC Classic Chemistry Experiments (1995) Emulsions are mixtures of two immiscible liquids in which droplets of one are dispersed in a continuous phase of the other.Practical Chemistry (Nuffield Foundation/RSC).3.15 Nuclear magnetic resonance spectroscopy.3.14 Organic synthesis and analysis (A2).In a water-in-oil emulsion, the roles are switched. 2.6 Reactions of ions in aqueous solution When an emulsion is oil-in-water, oil is the dispersed phase that is distributed into the continuous phase, water.2.4 Properties of Period 3 elements and their oxides.1.11 Electrode potentials and electrochemical cells (Redox A2).1.10 Equilibrium constant Kc for homogeneous systems (Equilibrium A2).3.6 Organic analysis (AS): analytical techniques.1.7 Oxidation reduction equations (Redox AS).1.6 Chemical equilibria and Le Chatelier’s principle.C3.5 Production of ammonia (an example of a reversible reaction).C3.4 Further analysis and quantitative chemistry.C3.3 Calculating and explaining energy change.Cream gel transparent cosmetic serum oil sample texture with bubbles. C2.5 Exothermic and endothermic reactions Find Oil In Water Emulsion stock images in HD and millions of other royalty-free.Commonly used emulsifiers include egg yolk, or mustard. C2.3 Atomic structure, analysis and quantitative chemistry By vigorously mixing the emulsifier with the water and fat/oil, a stable emulsion can be made.C1.7 Changes in the Earth and its atmopshere.C1.5 Other useful substances from crude oil.


 0 kommentar(er)
0 kommentar(er)
